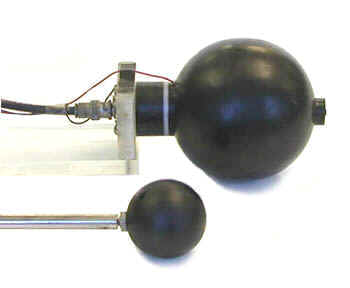
Ionization Chambers
Condenser Ion Chambers
-
Condenser Ionization Chamber for Measuring X-ray Emissions from Television Sets Condenser Ionization Chamber for Measuring X-ray Emissions from Television Sets

-
Unusual Condenser Ionization Chamber Unusual Condenser Ionization Chamber

-
Well-type Condenser Ionization Chamber Well-type Condenser Ionization Chamber

Ion Chambers for Cosmic Ray Studies
-
High Pressure Ionization Chamber High Pressure Ionization Chamber

-
Ionization Chamber Used at Robert Millikan's Laboratory for Cosmic Ray Studies Ionization Chamber Used at Robert Millikan's Laboratory for Cosmic Ray Studies

Ion Chambers for the Analysis of Radon in Breath Samples
Analysis of Radon in Breath Samples
By the end of the 1920s, the medical community had become convinced that the premature deaths of many of the radium dial painters could be attributed to the radium-226 that had been incorporated into their skeletons. Around 1930 or so, concerted efforts began to quantify the activities of the internally deposited radium. Such measurements were complicated by the fact that the Ra-226 decayed into the noble gas radon-222, a large percentage of which was eliminated from the body by exhalation.
Measurements of radium content in the body were performed in two ways.
- Analyzing the radon concentration in the exhaled breath. This was done by transferring a breath sample into an ionization chamber. Either the current or pulse rate from the chamber was then related to the radon concentration. The latter could then be used to determine the activity of the radium in the body that decayed to radon that was exhaled.
- Measuring the gamma ray emissions from the body, essentially the gamma rays emitted by the radon decay products lead-214 and bismuth-214. The emissions from the decay products could be used to determine the activity of the radium in the body that decayed to radon that was retained in the body. These measurements were performed with, electroscopes, Geiger-Mueller detectors or ionization chambers.
The first method did not reveal how much radium decayed to radon that was not exhaled and the second method did not tell how much radium decayed into radon that was exhaled. At the time, it was assumed that 45% of the radon-222 produced by the decay of Ra-226 was exhaled.
In 1941, the National Bureau of Standards Handbook No. 27 (NCRP Report No. 5) set the permissible body burden for radium-226 at 0.1 uCi, a value soon adopted by the ICRP. This was equated by the NBS and the Department of Labor to 1 pCi of Rn-222 per liter of exhaled air.
As a check on worker exposures, some facilities required regular (every four to six months) analyses of breath samples. If a worker exceeded the tolerance level, he or she was transferred to non-radioactive work for up to a month. This would usually result in the elimination of 50 to 90% of the radium body burden. The breath samples were collected at the facility but the actual analysis was performed at a laboratory with the specialized equipment and expertise. Robley Evans at MIT was the first to perform these analyses. Later, similar measurements were performed at the National Bureau of Standards and Fordham University (Victor Hess).
The first measurements of radon in breath that I know of were conducted at Standard Chemical Company in Pittsburg (ca. 1920), but this was done to assess radon turnover rates rather than quantify radium in the body.
References
- Evans, Robley, Radium Poisoning II The Quantitative Determination of the Radium Content and Radium Elimination Rate of Living Persons, Am. J. Roentgenol. And Radium Therapy, 37: 368-378, 1937.
- Evans, Robley, Protection of Radium Dial Workers and Radiologists from Injury by Radium, J. Ind. Hygiene and Tox. 25 (7): 253-269, 1943.
-
Ion Chamber for Radon Analysis in Breath Samples Ion Chamber for Radon Analysis in Breath Samples

-
Ionization Chamber of Robley Evans Ionization Chamber of Robley Evans

-
Ionization Chamber of Victor Hess Ionization Chamber of Victor Hess

Ionization Chambers
The essential components of the ionization chamber are its two collecting electrodes: the anode and cathode (the anode is positively charged with respect to the cathode). In most cases, but not all, the outer chamber wall serves as the cathode. The potential difference between the anode and cathode is often in the 100 to 500 volt range. The most appropriate voltage depends on a number of things such as the chamber size (the larger the chamber, the higher the required voltage).
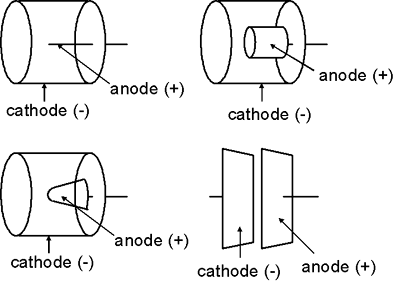
The shape of the electrodes in an ionization chamber are more variable than those of a Geiger-Mueller detector or proportional counter. In general, the outer chamber wall (the cathode) is a cylinder or sphere while the anode is usually rod-shaped. Nevertheless, the anode might take other shapes, e.g., a cylinder or cone. In some cases, the two electrodes might even be flat parallel plates.
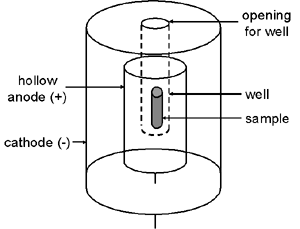
Another common type of ionization chamber is the well detector in which the outer chamber wall projects down inside a hollow tubular anode. This greatly increases the system’s sensitivity because the sample can be positioned in the center of the chamber.
The presence of radiation causes charged particles to traverse the gas inside the ionization chamber. These charged particles might be alpha or beta particles from a radioactive sample (if they have sufficient energy to penetrate the detector wall).
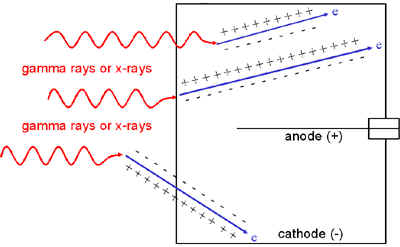
Alternatively, the charged particles might be electrons to which gamma rays or X-rays have transferred energy via the photoelectric effect, Compton scattering or pair production. Most of these gamma ray or X-ray interactions occur in the wall of the detector, but some also occur in the chamber fill gas. If the chamber wall is thin enough, these electrons might even originate in gamma ray or X-ray interactions outside the chamber.
The movement of the charged particles through the chamber ionizes the atoms or molecules of the gas, i.e., creates ion pairs. For example, this ionization process might involve an electron being stripped away from a nitrogen molecule—the freed electron would be the negative member of the ion pair and the positively charged nitrogen molecule would be the positive member of the ion pair.
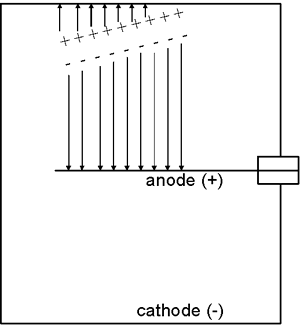
The electric field created by the potential difference between the anode and cathode causes the negative member (electron) of each ion pair to move to the anode while the positively charged gas atom or molecule is drawn to the cathode. The movement of the ions to the collecting electrodes results in an electronic pulse. Since these pulses are usually too small to be detected, the most common approach is to measure the ion chamber’s current which is produced by many radiation interactions in the detector and is more easily measured than the individual pulses.
-
Frisch Grid Ionization Chamber Frisch Grid Ionization Chamber

-
Herb Parker's Free Air Ionization Chamber for X-Ray Measurements Herb Parker's Free Air Ionization Chamber for X-Ray Measurements

-
Ionization Chamber of Gino Failla for Beta Measurements Ionization Chamber of Gino Failla for Beta Measurements

-
Jordan Model CG 50 Ion Chamber Jordan Model CG 50 Ion Chamber

-
NBS 4 pi Well Ionization Chamber NBS 4 pi Well Ionization Chamber

-
NBS Ionization Chamber for Beta Emitters NBS Ionization Chamber for Beta Emitters
-
Predecessor to the Reuter-Stokes RS 111 Pressurized Ion Chamber Predecessor to the Reuter-Stokes RS 111 Pressurized Ion Chamber

Miscellaneous Ion Chambers
-
Cary Vibrating Reed Electrometer with Ionization Chamber Cary Vibrating Reed Electrometer with Ionization Chamber

-
Shonka Tissue Equivalent Ion Chambers Shonka Tissue Equivalent Ion Chambers