Ionization Chambers
The essential components of the ionization chamber are its two collecting electrodes: the anode and cathode (the anode is positively charged with respect to the cathode). In most cases, but not all, the outer chamber wall serves as the cathode. The potential difference between the anode and cathode is often in the 100 to 500 volt range. The most appropriate voltage depends on a number of things such as the chamber size (the larger the chamber, the higher the required voltage).
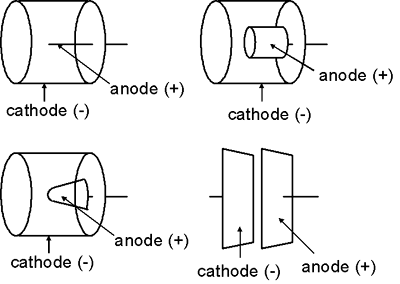
The shape of the electrodes in an ionization chamber are more variable than those of a Geiger-Mueller detector or proportional counter. In general, the outer chamber wall (the cathode) is a cylinder or sphere while the anode is usually rod-shaped. Nevertheless, the anode might take other shapes, e.g., a cylinder or cone. In some cases, the two electrodes might even be flat parallel plates.
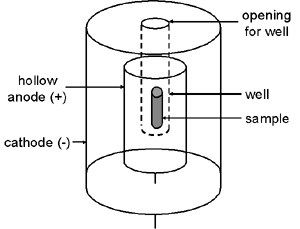
Another common type of ionization chamber is the well detector in which the outer chamber wall projects down inside a hollow tubular anode. This greatly increases the system’s sensitivity because the sample can be positioned in the center of the chamber.
The presence of radiation causes charged particles to traverse the gas inside the ionization chamber. These charged particles might be alpha or beta particles from a radioactive sample (if they have sufficient energy to penetrate the detector wall).
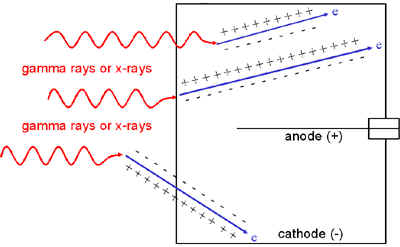
Alternatively, the charged particles might be electrons to which gamma rays or X-rays have transferred energy via the photoelectric effect, Compton scattering or pair production. Most of these gamma ray or X-ray interactions occur in the wall of the detector, but some also occur in the chamber fill gas. If the chamber wall is thin enough, these electrons might even originate in gamma ray or X-ray interactions outside the chamber.
The movement of the charged particles through the chamber ionizes the atoms or molecules of the gas, i.e., creates ion pairs. For example, this ionization process might involve an electron being stripped away from a nitrogen molecule—the freed electron would be the negative member of the ion pair and the positively charged nitrogen molecule would be the positive member of the ion pair.
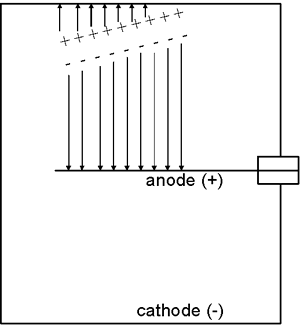
The electric field created by the potential difference between the anode and cathode causes the negative member (electron) of each ion pair to move to the anode while the positively charged gas atom or molecule is drawn to the cathode. The movement of the ions to the collecting electrodes results in an electronic pulse. Since these pulses are usually too small to be detected, the most common approach is to measure the ion chamber’s current which is produced by many radiation interactions in the detector and is more easily measured than the individual pulses.
-
Frisch Grid Ionization Chamber Frisch Grid Ionization Chamber

-
Herb Parker's Free Air Ionization Chamber for X-Ray Measurements Herb Parker's Free Air Ionization Chamber for X-Ray Measurements

-
Ionization Chamber of Gino Failla for Beta Measurements Ionization Chamber of Gino Failla for Beta Measurements

-
Jordan Model CG 50 Ion Chamber Jordan Model CG 50 Ion Chamber

-
NBS 4 pi Well Ionization Chamber NBS 4 pi Well Ionization Chamber

-
NBS Ionization Chamber for Beta Emitters NBS Ionization Chamber for Beta Emitters
-
Predecessor to the Reuter-Stokes RS 111 Pressurized Ion Chamber Predecessor to the Reuter-Stokes RS 111 Pressurized Ion Chamber

