Radioactive Consumer Products
Everything is radioactive. The question is, "How radioactive?"
As is often necessary, the scope of this category is being stretched—a number of the items shown here were never sold to the public as consumer products.
Ceramics
General Information about Uranium in Ceramics
Paul Frame, Oak Ridge Associated Universities
General
Ordinary ceramics often contain elevated levels of naturally occurring radionuclides, e.g., K-40 and the various members of the uranium and thorium decay series. Because of this, health physicists who are conducting radiation surveys expect to see higher readings when they are making measurements over ceramic tiles and similar materials. Sometimes the higher readings are due to uranium in the glaze, sometimes they are due to the radionuclides in the clay that was used to produce the ceramic. For example, I was once at a truck weigh station when a vehicle carrying toilets set off a radiation monitor. As another example, health physicists at Oak Ridge National Laboratory reported excessively high readings while surveying newly purchased urinals for the men’s restrooms. Perhaps they should have been spelt “uranyls?”
Ceramics can be particularly radioactive if some compound of uranium (e.g., uranium oxide, sodium urinate) has been used to impart color (e.g., orange-red, green, yellow, black) to the glaze. It is widely known that uranium was used in the glaze of orange-red Fiesta dinnerware, but uranium glazes have also been used other types of ceramics: wall and floor tiles, pottery, laboratory ceramics, etc. The glaze can serve two functions: it provides color, and it seals the ceramic.
The use of uranium in ceramic glazes ceased during World War II and didn’t resume until 1959. In 1987, NCRP Report 95 indicated that no manufacturers were using uranium-glaze in dinnerware.
Potential Doses
Although the uranium in the glaze emits gamma rays, alpha particles, and beta particles, the gamma and alpha emissions are weak. The beta particles are the easiest to detect, and they are also responsible for the bulk of the radiation exposure to those handling ceramics that employ a uranium glaze.
NCRP Report 95 reported the following measurements for dinnerware employing uranium glazes: 0.2 to 20 mrad per hour on contact as measured using film badges.
Measurements of the radioactivity of ceramics that are reported in mR/hr are difficult to interpret unless it is known whether or not the detector was responding to beta particles. The exposure rate, in mR/hr, is defined for x-rays or gamma rays. It is not defined for betas. If a measurement is reported in mR/hr, the detector should not be responding to beta particles.
NUREG/CRCP-0001 reported a measurement of approximately 0.7 mR/hr at 25 cm from a Fiesta red dinner plate. It also reported the results of an Oak Ridge National Laboratory analysis that predicted 34.4 mrem/year to a dishwasher at a restaurant using ceramic plates containing 20% uranium in the glaze, 7.9 mrem/year to the waiters, and 0.2 mrem to a patron for a four hour exposure.
It is likely that the major health issue associated with this dinnerware is not the radiation exposures, but the ingestion of uranium or other metals that have leached into food or drink in contact with the dinnerware. One FDA study measured 1.66 x 10-5 uCi/ml in a 4 % acetic acid solution in contact with the ceramic dinnerware for 50 hours—this exceeded the ICRP’s maximum permissible concentration (MPC).
One common misconception is that a uranium-containing glaze can be a source of radon-222. This is incorrect because the glaze contains chemically purified uranium, not the complete uranium series. Chemically purified uranium contains: U-238 plus its two short-lived decay products, Th-234 and Pa-234m; U-234; and U-235 plus its decay product Th-231. Since Ra-226 is not present, there is no radon (Rn-222) production. It is true that the clay used to produce the body of the ceramic (rather than the glaze) can be a source of radon, but this is true for all ceramics, with or without a uranium glaze.
Pertinent Regulations
10 CFR 40.13 Unimportant quantities of source material.
(c) Any person is exempt from the regulation in this part and from the requirements for a license set forth in section 62 of the Act to the extent that such person receives, possesses, uses, or transfers:
(2) Source material contained in the following products: (i) Glazed ceramic tableware, provided that the glaze contains not more than 20 percent by weight source material; (ii) Piezoelectric ceramic containing not more than 2 percent by weight source material; (iii) Glassware containing not more than 10 percent by weight source material; but not including commercially manufactured glass brick, pane glass, ceramic tile, or other glass or ceramic used in construction;
Reference
Harry McMaster. Earthenware Dishes and Glaze Therefor. U.S. Patent No. 1,890,297, December 6, 1932.
-
Cloisonné Jewelry Cloisonné Jewelry

-
Fiestaware Fiestaware

-
Radioactive Tiles Radioactive Tiles

-
Uranium Containing Dentures Uranium Containing Dentures
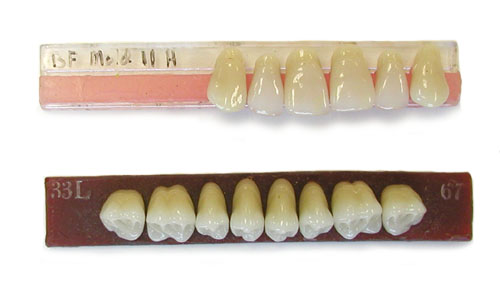
-
Uranium Containing Spot Plate Uranium Containing Spot Plate

Depleted Uranium
-
Depleted Uranium Dice Depleted Uranium Dice

-
Depleted Uranium Frizzen Depleted Uranium Frizzen

-
Depleted Uranium Penetrator Rounds Depleted Uranium Penetrator Rounds

Food
-
Brazil Nuts Brazil Nuts

-
Check·Up Gum Check·Up Gum

-
Low Sodium Salt Substitutes Low Sodium Salt Substitutes

Glass
-
Uranium Containing Marble Uranium Containing Marble

-
Vaseline and Uranium Glass Vaseline and Uranium Glass

Miscellaneous
-
Antidiarrhea Medications Antidiarrhea Medications

-
Cat Litter Cat Litter

-
Electron Tubes Electron Tubes

-
Fertilizer Fertilizer

-
Glossy Paper Glossy Paper

-
Jewelry Made from Radium Dial Watches Jewelry Made from Radium Dial Watches

-
Potassium Chloride Water Softener Salt Potassium Chloride Water Softener Salt

-
Radioactive Spark Plugs Radioactive Spark Plugs

-
Radioactive Tape Dispenser Radioactive Tape Dispenser

-
Radio Golf Ball Radio Golf Ball

-
Smoke Detector Smoke Detector

-
Static Eliminators Static Eliminators

-
Uranium Containing Pencil Uranium Containing Pencil

Products Containing Thorium
General Information about Thorium
Paul Frame, Oak Ridge Associated Universities
Thorium is a dense, insoluble metal that was discovered in 1828 by the Swedish chemist, Jons Berzelius. Almost all thorium is obtained from the mineral monazite.
The members of the Thorium decay series and their half-lives are as follows:
| Th-232 | 1.4 x 1010 years |
| Ra-228 | 5.7 years |
| Ac-228 | 6.1 hours |
| Th-228 | 1.9 years |
| Ra-224 | 3.6 days |
| Rn-220 | 55 seconds |
| Po-216 | 0.1 seconds |
| Pb-212 | 10.6 hours |
| Bi-212 | 60.6 minutes |
| Po-212 | 3 microseconds |
| Tl-208 | 3.1 minutes |
The gamma exposure rate associated with something containing thorium (e.g., welding rods, thoriated lenses, parts made from magnesium-thorium alloys) changes over time. How the exposure rate changes depends on how many years it was after the purification of the thorium that the item was produced.
Chemically purified thorium is a mix of Th-232 and Th-228 and Th-230. The small quantity of Th-230 is of little consequence since it does not emit gamma rays (to any significant extent) and because there is no significant ingrowth of its decay products.
After purification of the thorium, the ingrowth of the decay products of Th-232 and Th-228 is a somewhat complex process although it is reasonably accurate to assume that the decay products of Th-228 (Ra-224, Rn-220, Po-216, Pb-212, Bi-212, Po-212 and Tl-208) are always present at the same activity as the Th-228.
During the first five years after the purification of thorium, the change in the exposure rate is minimal, although there is a slight decrease in the first two years. Afterwards, the exposure rate increases. At the 20 year mark, all the members of the series will be very close to equilibrium (present at the same activities) and the exposure rate will be approximately two and a half times the exposure rate during the first five years. No further change will occur.
-
Incandescent Gas Lantern Mantles Incandescent Gas Lantern Mantles

-
Magnesium-Thorium Alloy Magnesium-Thorium Alloy

-
Thoriated Camera Lens Thoriated Camera Lens

-
Thorium Containing Welding Rod Thorium Containing Welding Rod

The following are not in the ORAU Collection, but are shown for informational purposes
-
Lightning Rod/Arrestor Lightning Rod/Arrestor

-
Radium Containing Industrial Smoke Detector Radium Containing Industrial Smoke Detector

