When I was diagnosed with stage-3b rectal cancer in 2012, radiation combined with oral chemotherapy was prescribed as the first phase of a three-phase treatment protocol. The other phases included surgery and infused chemotherapy.
Cancer in my rectum meant that all 35 radiation treatments were aimed where the sun doesn’t shine. In addition to the treatment itself, I underwent several CT and PET scans to help determine where exactly the cancer was located, what stage cancer we were treating, and then whether treatment was working.
I’m not sure how much total radiation I was exposed to during cancer treatment, but Dray Gentry, a clinical physicist working on ORAU’s radiation epidemiology team, hopes to help answer that question for cancer patients.
Gentry, a Ph.D. candidate at the University of Tennessee, is the primary investigator for an ORAU-Directed Research and Development Grant project that has two research aims. The first is to determine the feasibility of using artificial intelligence to speed up the process of identifying chromosomal abnormalities in the Radiation Emergency Assistance Center/Training Site Cytogenetic Biodosimetry Laboratory, which is part of the Oak Ridge Institute for Science and Education that ORAU manages for the U.S. Department of Energy. The second is to help determine the total dose cancer patients are exposed to during treatment.

Dray Gentry, University of Tennessee Ph. D. candidate, uses AI to improve efficiency in cancer treatment.
“Radiation is one of those weird things where you can’t see it. You can’t smell it. It’s just something that’s there,” Gentry said, pointing to the challenges in measuring and monitoring radiation exposure. “Then there’s detecting it. We don’t have an internal radiation detection monitor on us [which means there’s not an easy way to determine exposure level].”
Cytogenetic biodosimetry and AI
Scientists can estimate the amount of radiation someone has been exposed to by counting the number of dicentric chromosomes in a blood sample, though.
“We’re doing something called cytogenetic biodosimetry,” Gentry said. “If you break that down, cytogenetic is “cyto,” which means cell, and “genetic” is referring to DNA. Biodosimetry is measuring radiation dose.”
He explained that when you look at chromosomes, they normally form an “X,” and you can see them under a microscope. Chromosomes that are exposed to radiation break apart. In an ideal situation, when they break apart, they re-form into their original “X,” but sometimes they reform as two “Xs” stacked on top of each other, having two pinch points, called centromeres, instead of just one. These malformations are called dicentric (having two centromeres) chromosomes; and when you have a lot of them, it can be correlated to the amount of radiation a person has been exposed to.
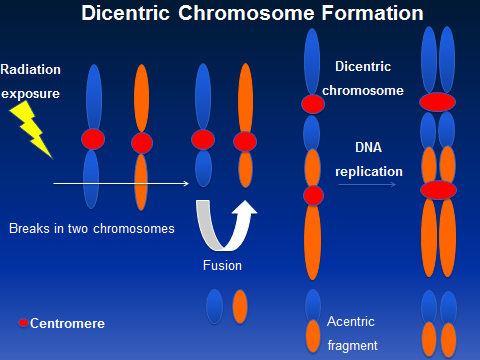
Radiation can cause chromosomes to break. Sometimes they reform as two “Xs” rather than one.
“We can count the number of dicentric chromosomes to get an estimation of the radiation dose someone has received,” Gentry said.
While cytogenetic biodosimetry is the gold standard for determining radiation dose exposure, it is highly time-consuming. In the event of a mass exposure event, like a radiation leak or detonation of a dirty bomb, conducting large-scale biodosimetry quickly will be virtually impossible.
“That’s where the AI model comes in,” Gentry said. “We wrote an AI model to look at these images and count the number of dicentric chromosomes in them.”
Gentry teamed up with Chet Ramsey, adjunct professor in the Department of Nuclear Engineering at the University of Tennessee, which is a member of the ORAU University Consortium. developed a website where images from blood samples can be uploaded and scanned using AI. Using the process they developed, scanning for dicentric chromosomes and counting the number of them in a given sample took about four seconds.
“We were really pleased with the results,” he said.
Estimating total radiation dose in cancer patients
The second aim of Gentry’s project is to determine the total biological radiation dose in patients treated for cancer with radiation therapy, as well as all the CT scans, PET scans and other tests that expose patients to radiation.
Gentry explained that the planned dose is the amount of radiation a patient is anticipated to be exposed to during radiation therapy. In my case, that was 35 rounds of radiation. Biological dose would also include radiation exposure that occurs during pre-treatment simulations as well as CT and other scans that occur during treatment.
How much radiation are cancer patients exposed to during treatment? ODRD project is seeking answers. (Getty stock image)
“That’s a lot of dose,” he said, referring to the total dose from the combination of treatment and additional scans. “But it’s ignored because it’s seen as trivial compared to the dose we’re giving with the therapy,” Gentry explained.
He wondered how an actual biological dose could be determined for a patient.
“We can take their blood before treatment and after treatment and then get a biological dose compared to their planned dose,” he said.
What’s next?
Gentry said he and Ramsey are pleased with the results of their project and are in the final stages of writing a paper for publication in a peer-reviewed journal. Additionally, they have begun work on a project with the Thompson Proton Center, part of Covenant Health’s Thompson Cancer Survival Center in East Tennessee. The project’s goal is to determine how proton therapy, a type of radiation therapy that uses high-energy protons to treat cancer, affects cytogenetics.
Understanding how protons impact cytogenetics is an important initiative of the National Cancer Institute, and Gentry’s research has the potential to keep ORAU on the cutting edge of cancer research.
What are ODRD grants?
Let’s take a step back for a moment and talk about ODRD grants.
ODRD is a research and development program that supports collaborations between ORAU researchers and faculty at academic institutions that are part of ORAU’s University Consortium. Led by ORAU subject matter experts and leveraging the talents and expertise of our member universities, ODRD projects strengthen and expand the scientific and technical capabilities of both parties.
In this case, Gentry joined forces with Chet Ramsey, adjunct professor in the Department of Nuclear Engineering at the University of Tennessee, which is a member of the ORAU University Consortium.
At its core, ODRD provides a path for funding innovative research-based approaches/solutions that fall within the intersection of ORAU’s capabilities and our member universities’ research interests. Ideally, ODRD-funded projects result in proposals that can generate new, sponsored research that is jointly performed by ORAU and partner universities.
ODRD supports university-engaged, applied research while increasing the potential for significant external research funding and strengthening ORAU’s ability to address current and future customer needs.